Azathioprine Trial Eligibility Checker
Check Your Eligibility for Azathioprine Trials
Answer a few questions to determine if you might qualify for current azathioprine clinical trials. This is not a definitive assessment - discuss with your physician and clinical trial coordinator.
Eligibility Assessment
This tool provides a general eligibility assessment based on standard clinical trial criteria. It is not a substitute for medical advice. Discuss your eligibility with your physician and clinical trial coordinator.
When you hear the words Azathioprine clinical trials, you might picture a sterile lab or a long list of medical jargon. In reality, these studies are a gateway for patients to help shape the next wave of treatments for autoimmune diseases and organ transplant care. Below you’ll learn exactly what azathioprine is, how clinical trials are organized, and what steps you can take to become a participant.
What Is Azathioprine?
Azathioprine is an oral immunosuppressive medication used to treat autoimmune disorders and prevent organ transplant rejection. It works by slowing down the activity of white blood cells, which helps curb the body’s own attack on healthy tissue. Doctors prescribe it for conditions such as inflammatory bowel disease (IBD), rheumatoid arthritis, systemic lupus erythematosus, and for patients who have received a kidney, liver, or heart transplant. Because it modulates the immune system, azathioprine can have side effects ranging from mild nausea to more serious blood count changes, which is why ongoing research is essential.
Why Clinical Trials Matter for Azathioprine
Clinical trials provide the evidence base that determines whether a drug like azathioprine is safe, effective, and ready for broader use. They also explore new dosing strategies, combination therapies, and identify patient groups that benefit most. Without trials, physicians would rely on outdated information, and patients would miss out on potential advances.
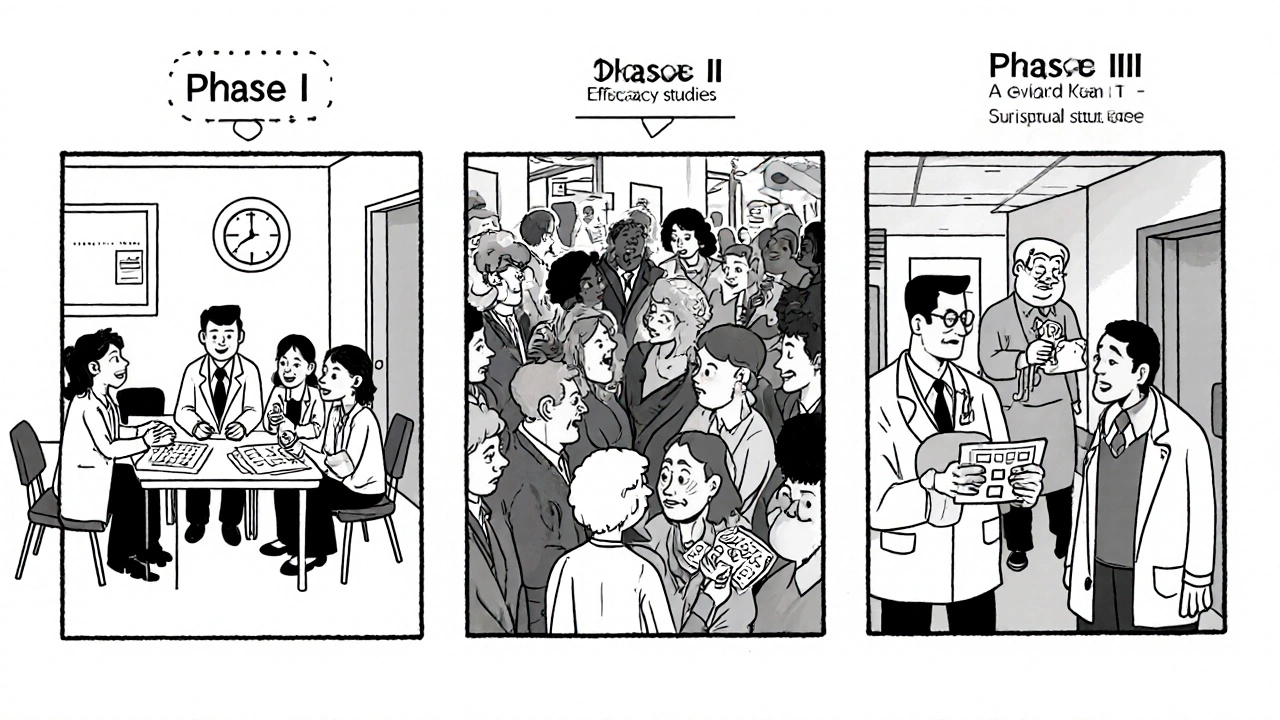
How Clinical Trials Are Structured
Every trial follows a strict roadmap overseen by regulatory bodies such as the FDA (U.S. Food and Drug Administration, the agency that reviews safety and efficacy of drugs). The roadmap is divided into phases, each answering specific questions about the drug.
| Phase | Primary Goal | Typical Sample Size | Duration | Focus for Azathioprine |
|---|---|---|---|---|
| Phase I | Safety and dosage | 20-80 healthy volunteers or patients | Months | Identify safe starting dose, monitor blood counts |
| Phase II | Efficacy and side‑effect profile | 100-300 patients with target disease | 6-12 months | Test azathioprine in IBD or rheumatoid arthritis |
| Phase III | Confirm effectiveness, compare with standard care | 1,000-3,000 patients | 1-3 years | Head‑to‑head against newer biologics, assess long‑term safety |
Eligibility: Who Can Join an Azathioprine Trial?
Eligibility criteria are designed to protect participants and ensure reliable data. Common requirements include:
- Age range (often 18‑65, though pediatric studies exist for IBD)
- Confirmed diagnosis of the condition under study
- Stable baseline labs (especially liver function and blood counts)
- No recent use of conflicting immunosuppressants
- Ability to attend scheduled visits and adhere to medication regimen
Each trial’s Informed Consent (a legal document outlining study purpose, procedures, risks, and rights) will spell out the exact criteria, so it’s crucial to read it carefully.
How to Find and Enroll in a Trial
Patient recruitment is coordinated by research centers, hospitals, and sometimes national databases. Here’s a step‑by‑step guide:
- Search reputable registries such as ClinicalTrials.gov (U.S. National Library of Medicine’s trial database) or your country’s health ministry portal.
- Filter results by “Azathioprine,” disease area (e.g., ulcerative colitis), and location.
- Read the study brief, paying attention to phase, duration, and required visits.
- Contact the trial’s coordinator using the phone or email listed; ask about screening appointments.
- Undergo screening labs and medical history review to confirm eligibility.
- If approved, sign the informed consent form and receive the study medication.
- Follow the visit schedule, report any side effects promptly, and keep a personal log.
Many trials also offer travel reimbursements or modest compensation for time, which can offset the logistical burden.
Potential Benefits and Risks
Joining a trial is a personal decision that balances hope for a better outcome against possible uncertainties.
- Benefits: Access to cutting‑edge dosing regimens, close monitoring by specialist physicians, and contribution to science that could help future patients.
- Risks: Unknown side effects, placebo exposure (in randomized controlled trials), extra clinic visits, and the possibility that the experimental approach proves less effective than standard care.
Most azathioprine studies include rigorous Adverse Event Monitoring (continuous safety checks for any negative reactions), so participants are not left in the dark.
Current Azathioprine Trials (2025 Snapshot)
As of October 2025, several notable studies are recruiting:
- Trial A: Phase II multicenter study evaluating low‑dose azathioprine combined with probiotic therapy for ulcerative colitis remission.
- Trial B: Phase III randomized trial comparing azathioprine to a newer JAK inhibitor in rheumatoid arthritis patients who have failed methotrexate.
- Trial C: Pediatric Phase I safety trial of azathioprine in children under 12 with early‑onset lupus.
These trials are hosted at major academic hospitals in the United States, Europe, and Australia, and many accept international participants via tele‑medicine follow‑ups.

Tips for a Smooth Trial Experience
- Organize your medical records. Bring a complete list of current meds, past lab results, and vaccination history.
- Ask about the schedule. Knowing how many visits and blood draws are required helps you plan work or family commitments.
- Maintain open communication. Report any new symptoms immediately; study staff can adjust dosing to keep you safe.
- Use a medication diary. Tracking doses, side effects, and daily symptoms provides valuable data for the researchers.
- Know your rights. You can withdraw at any time without penalty, and the informed consent form will detail how your data is protected.
Key Takeaways
- Azathioprine is a long‑standing immunosuppressant used for autoimmune diseases and organ transplant maintenance.
- Clinical trials are the only way to refine dosing, test combos, and improve safety for future patients.
- Eligibility hinges on diagnosis, stable labs, and ability to follow study procedures; consent forms spell out all details.
- Finding a trial involves searching registries, contacting coordinators, and completing screening.
- Participation offers close medical supervision and a chance to influence new therapies, while risks are mitigated by strict monitoring.
Frequently Asked Questions
Can I join a trial if I’m already taking azathioprine?
Yes, many studies specifically enroll patients who are on stable azathioprine doses to test new combinations or dose adjustments. The trial will assess whether your current regimen meets their inclusion criteria.
What happens if I receive a placebo?
In a randomized controlled trial, some participants receive a placebo to compare outcomes fairly. You’ll still receive the same level of monitoring and care, and you can withdraw at any point.
How are side effects tracked?
Trial staff perform regular lab tests (CBC, liver enzymes) and use standardized questionnaires to capture symptoms. Any serious adverse event is reported to the study’s safety monitoring board immediately.
Do I have to travel far to participate?
Some trials offer remote visits or partner sites closer to your home. Check the trial’s description for travel assistance options.
Will my personal health data be kept private?
Yes. Data is de‑identified and stored on secure servers. The informed consent form explains the exact privacy safeguards used.
Whether you’re living with ulcerative colitis, rheumatoid arthritis, or have received a transplant, taking part in an azathioprine trial can be a powerful way to help yourself and future patients. Use the steps above to explore opportunities, ask the right questions, and make an informed choice.




Comments
Dante Russello
October 23, 2025
Great overview of azathioprine trials; the step‑by‑step guide is especially handy, and the table makes the phase differences crystal clear. I appreciate how you highlighted both the benefits-like close monitoring-and the potential risks, because that balance is crucial for anyone considering enrollment. The tips on organizing medical records and using a medication diary are practical, and many patients overlook them. Also, the mention of travel reimbursements can be a game‑changer for folks living far from study sites. Overall, a thorough resource that demystifies the whole process.
James Gray
October 23, 2025
Totally agree-this is super helpful!! I love the part about remote visits, it makes joining a trial way easier for people who cant travel far. Keep spreading the word, man, cuz the more ppl know, the better.
Scott Ring
October 23, 2025
Reading this made me realize how much groundwork goes into each trial before a drug even reaches patients. The breakdown of eligibility criteria is spot‑on, especially the emphasis on stable labs and medication history. It’s also reassuring to see that informed consent forms are emphasized so heavily; consent should never be a rushed signature. For anyone on a stable azathioprine regimen, this article provides a clear path to explore new combos safely.
Shubhi Sahni
October 23, 2025
Indeed, the depth of information presented here is remarkable, and it serves as an excellent primer for patients who are contemplating participation in clinical research; each section builds upon the previous, creating a cohesive narrative that demystifies a complex process. First, the historical context of azathioprine underscores its longstanding role in immunosuppression, reminding readers that the drug is not a newcomer, but rather a well‑studied backbone of therapy; this foundation helps set realistic expectations for trial outcomes. Second, the clear delineation of trial phases-Phase I focusing on safety, Phase II on efficacy, and Phase III on comparative effectiveness-provides a roadmap that many patients find otherwise opaque. Third, the eligibility checklist, with its emphasis on age range, confirmed diagnosis, and stable baseline labs, acts as a self‑screening tool, empowering individuals to assess their own suitability before reaching out to a coordinator. Fourth, the step‑by‑step guide to locating trials on ClinicalTrials.gov or national portals bridges the gap between curiosity and actionable steps, turning abstract desire into concrete action. Fifth, the practical advice on organizing medical records, maintaining a medication diary, and communicating openly with study staff cannot be overstated; these habits not only facilitate smooth enrollment but also enhance overall care. Sixth, the discussion of potential benefits-such as access to cutting‑edge dosing regimens and close specialist monitoring-highlights the reciprocal nature of clinical research, where participants contribute to science while receiving heightened attention. Seventh, the balanced presentation of risks, including unknown side effects and the possibility of receiving placebo, fosters informed decision‑making, ensuring that enthusiasm does not eclipse caution. Eighth, the mention of travel reimbursements and remote visit options acknowledges socioeconomic barriers, reinforcing the inclusive ethos of modern trials. Ninth, the snapshot of ongoing azathioprine studies in 2025, spanning ulcerative colitis, rheumatoid arthritis, and pediatric lupus, illustrates the breadth of investigation and the diverse patient populations that can benefit. Tenth, the emphasis on data privacy, with de‑identified storage and secure servers, alleviates common concerns about confidentiality. Eleventh, the reminder that participants can withdraw at any time without penalty empowers patients with agency, a cornerstone of ethical research. Twelfth, the array of resources provided-including links to trial registries and relevant literature-offers a springboard for deeper exploration. Thirteenth, the encouraging tone throughout the article motivates readers to take the first step, whether that be a phone call to a coordinator or a simple online search. Fourteenth, the inclusion of real‑world tips, such as checking for companion sites closer to home, demonstrates an understanding of logistical realities. Finally, the overall structure, punctuated by clear headings, bullet points, and tables, makes the information digestible, preventing overwhelm and fostering confidence in the reader's ability to navigate the clinical trial landscape.
Danielle St. Marie
October 24, 2025
Honestly, most laypeople have no clue how rigorous these azathioprine trials are-it’s not some wild west experiment, it’s a meticulously regulated process. 🇺🇸 If you want real progress, you need to back scientifically sound studies, not fringe “miracle cure” fads. 😤
keerthi yeligay
October 24, 2025
Thx 4 the insight, i think its valuble but whys the placebo arm so big?
Peter Richmond
October 24, 2025
The article effectively balances educational content with actionable guidance, making it a valuable reference for both clinicians and prospective participants seeking clarity on trial enrollment procedures.
Bonnie Lin
October 24, 2025
Glad you found it helpful.
Write a comment